Understanding the Science of Ocean and Coastal Acidification
On this page:
Ocean Acidification
For more in-depth information on ocean and coastal acidification science, see:
- National Academy of Science: Ocean Acidification
- National Academy of Science: Ocean Acidification Starting with the Science
- NOAA Ocean Acidification Program: What is Ocean Acidification
The Global Carbon Cycle
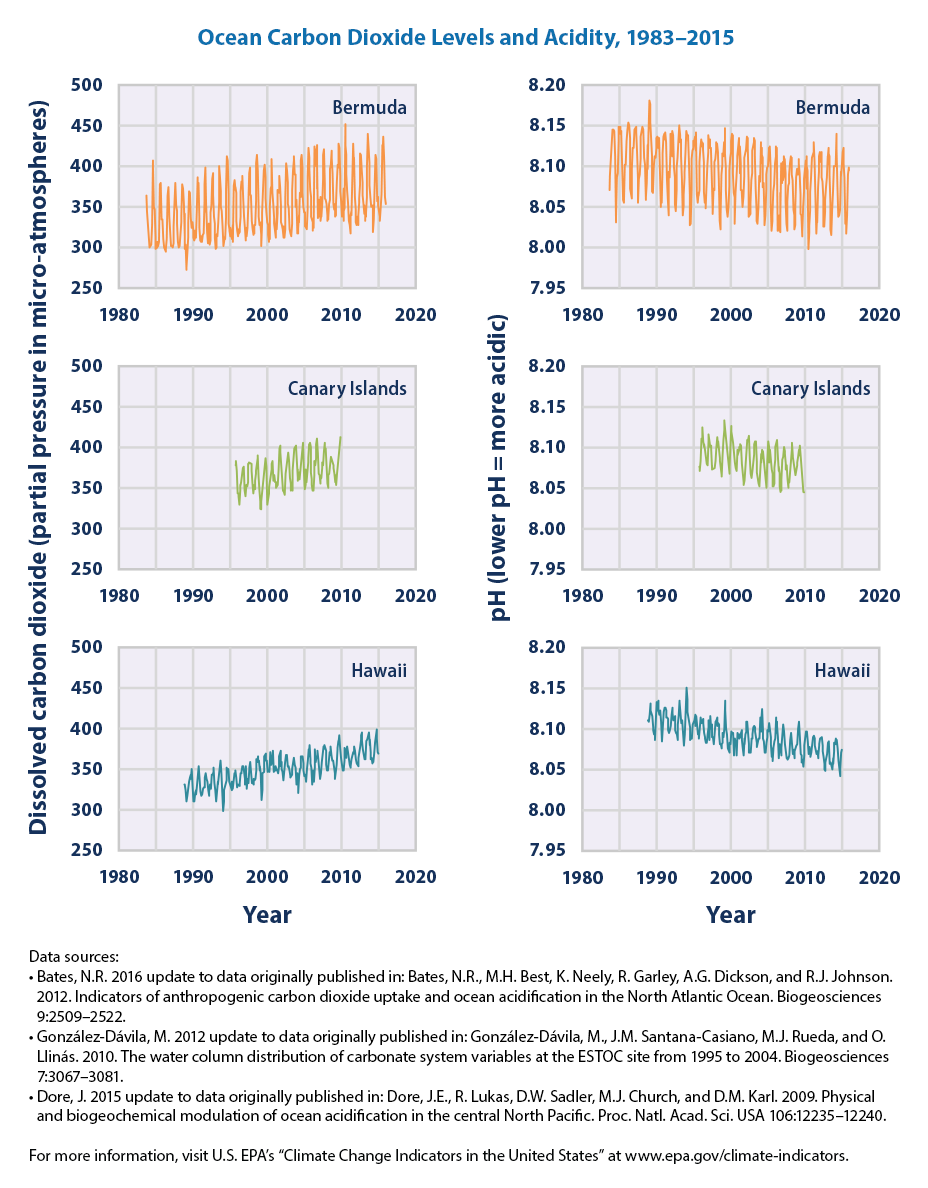
The amount of carbon dioxide has fluctuated slightly during the past 10,000 years. However, since the 1700s there has been a dramatic increase in the concentration of CO2 in the atmosphere. The post-1700s to current day concentrations of CO2 have exceeded known amounts of atmospheric carbon dioxide from the past 800,000 years. Changes to atmospheric carbon dioxide also impacts oceanic chemistry which includes acidity levels. The ocean readily absorbs dissolved carbon dioxide, which in turn increases the acidity.
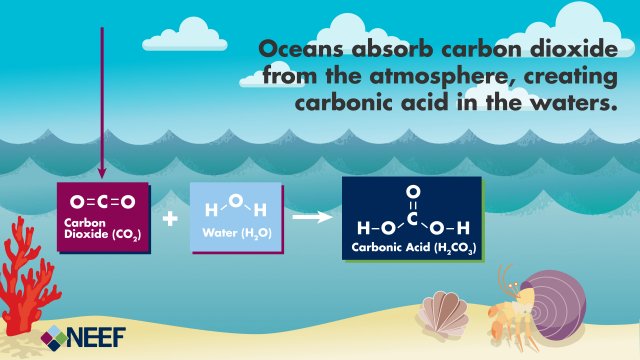
Dissolved Carbon Dioxide: Gases in Liquid?
Carbon Dioxide Imparts Acidity: Transformations of Carbon Dioxide in Water
Once carbon dioxide dissolves in water, it reacts with water molecules to form carbonic acid . Carbonic acid can be further transformed to bicarbonate and carbonate ions. These four different forms of carbon (dissolved carbon dioxide, carbonic acid, bicarbonate, and carbonate) exist in balanced proportions in seawater. As more carbon dioxide is added to seawater, the balance shifts and the carbonate ion concentration decreases as it is transformed to bicarbonate due to increasing acidity.
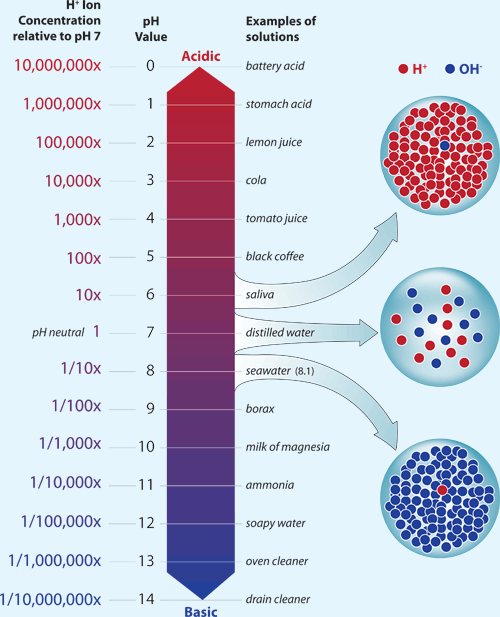
How Acidity is Measured: pH
The acidity of a liquid is reported as pHA representation of hydrogen ion concentration (molar hydrogen ion concentration to the negative base 10 logarithm) . The lower the pH value, the higher the acidity of a liquid. Solutions with low pH are acidic and solutions with high pH are basic (also known as alkaline).Prior to the 1700's, average ocean pH was about 8.2. Today, average ocean pH is about 8.1. This might not seem like much of a difference, but the relationship between pH and acidity is not direct. Each decrease of one pH unit is a ten-fold increase in acidity. This means that the acidity of the ocean today, on average, is about 25% greater than it was prior to the 1700's.
Acidity and Availability of Shell-forming Calcium Carbonate
Marine life uses carbonate from the water to build shells and skeletons. As seawater becomes more acidic, carbonate is less available for animals to build shells and skeletons. Under conditions of severe acidification, shells and skeletons can dissolve.
Coastal Acidification
Closer to Home: Coastal Acidification
Changing atmospheric composition, acid rain, and activities which cause nutrient pollution contribute to acidification in coastal waters. Excess nutrients contribute to acidification in coastal waters when peak and die.
Excess Nutrients Delivered Via Streams
The elements nitrogen and phosphorus are essential nutrients for living things. For this reason ,farmers homeowners, and gardeners supply nitrogen and phosphorus to crops, lawns, and gardens to stimulate plant growth. However, water can carry excess nutrients down streams and into coastal waters. Agricultural activities are a major source of nutrients to coastal waters, but other sources include