Registration Review Process
Registration Review Overview
EPA reviews each registered pesticide at least every 15 years to ensure that each pesticide can carry out its intended function(s) without creating unreasonable adverse effects to human health and the environment.
EPA always strives to base its decisions on the best available and sound science. However, science is constantly evolving, and new scientific information can come to light at any time and change our understanding of potential effects from pesticides. As part of registration review, EPA may identify additional data that may be useful for assessing a pesticide and require that it be submitted through a data call-in.
You can find the EPA’s planned registration review actions—including work plans, draft risk assessments, and decision documents—in the Registration Review Schedules.
Registration Review Process
While each pesticide review is unique, all pesticides go through the same basic registration review process.
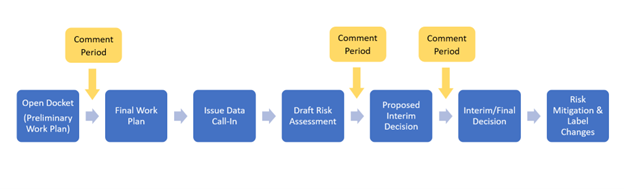
Note that whenever EPA determines there are urgent human or environmental risks from pesticide exposures that require prompt attention, the Agency will take appropriate regulatory action, regardless of the registration review status of the pesticide. EPA generally takes public comment on registration review cases at various points in the registration review process, shown in the graphic above. In response to comments submitted during the public comment period, EPA will incorporate new information and data into its risk assessment and regulatory documents as necessary. EPA will also provide a summary of responses to comments during the registration review process. Comments received on draft Biological Opinions are provided to the U.S. Fish and Wildlife Service and the National Marine Fisheries Service for incorporation into final Biological Opinions.
Learn more about each step below, or see EPA’s Registration Review Procedures.
Starting the Conversation: Preliminary and Final Work Plans
EPA initiates a registration review by establishing a public docket for a pesticide registration review case and opening the docket for public comment.
The docket contains a Preliminary Work Plan (PWP) summarizing information EPA has on the pesticide and the anticipated path forward. Among other things, the PWP includes:
- Facts about the pesticide and its current use and usage.
- Anticipated risk assessment and data needs.
- An estimated timeline for the review.
EPA publishes a notice in the Federal Register announcing the availability of the docket and providing the public with a comment period of at least 60 days. Anyone may submit data or information to the public docket. EPA considers the information received during the comment period and develops a Final Work Plan.
EPA also announces when a pesticide is no longer subject to registration review because the pesticide does not have any pesticide product registrations.
The registration review docket for each case will remain publicly accessible throughout the registration review, until all actions required in the final decision have been completed.
Links to registration review case dockets that have opened are available in Chemical Search.
Narrowing the Scope: Focus Meetings
To enhance transparency and involvement, EPA holds Focus Meetings for many pesticides going through registration review. Typically involving registrants and others early in the process, Focus Meetings are intended to address any areas of uncertainty such as unclear labels or missing studies that could affect EPA’s pesticide risk assessments and risk management decisions. By obtaining better information early in the process, EPA can narrow the scope of pesticide reevaluations to areas that pose real concerns, based on current data and use patterns.
Materials associated with Focus Meetings are available in the pesticide-specific registration review public dockets. When a Focus Meeting is held prior to the opening of a chemical-specific docket, materials are available in a special Focus Meetings docket, EPA-HQ-OPP-2012-0778
More information on Focus Meetings.
Gathering Information: Data Call-In and Risk Assessments
In conducting a pesticide's registration review, EPA will review available data and information. The Agency will:
Assess Changes since the Pesticide's Last Review
EPA will assess any changes that have occurred since the last registration decision to determine whether the pesticide still satisfies the statutory standard for registration. The Agency considers any new data or information on the pesticide and decide whether a new risk assessment or a new risk/benefit assessment must be conducted.
Conduct New Assessments as Needed
- If sufficient data or information are available, EPA will conduct the new risk assessment or risk/benefit assessment.
- If additional data or information are needed to conduct the review, EPA will issue a Data-Call In (DCI) notice to the registrant under the authority of FIFRA section 3(c)(2)(B).
Include the Public
EPA will generally make available for public review and comment a draft risk assessment for a pesticide if a new risk assessment has been conducted for registration review. The Agency also will announce the availability of a revised risk assessment. If risks of concern are identified, EPA may invite the public to submit suggestions for mitigating the risks.
Evaluate Effects on Endangered Species and Consult with our Regulatory Partners
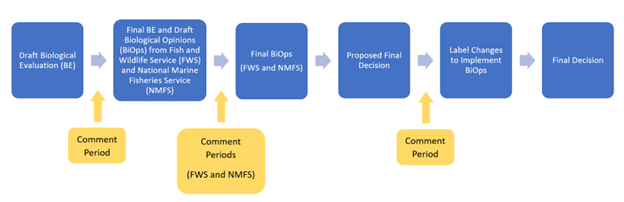
*This process likely occurs following the “Interim Decision” and before the “Final Decision” steps of the registration review process, but this is not always the case and may occur at other process steps.
When EPA reevaluates a pesticide in registration review, EPA has an obligation under the Endangered Species Act (ESA) to avoid jeopardizing federally listed threatened or endangered species and adversely modifying designated critical habitats when taking certain actions. EPA must consult with the U.S. Fish and Wildlife Service and the National Marine Fisheries Service (the Services) for any action that “may affect” listed species.
EPA’s ESA assessments for Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) actions begin with EPA considering whether the action may have any effect on a species or critical habitat. If EPA finds “no effect,” no further ESA analysis is needed. If EPA finds “may affect,” the Agency further assesses whether the pesticide’s use is either “not likely to adversely affect” (NLAA) or “likely to adversely affect” (LAA) the species or designated critical habitat, which in turn dictates whether formal or informal consultation with the Service(s) is required. For LAA determinations, EPA also may assess the potential likelihood of future jeopardy or adverse modification in its effects determination, consistent with 50 C.F.R. § 402.40(b)(1).
Where appropriate, EPA may include its effects determinations in a biological evaluation (BE), which also includes EPA’s analysis of the effects of a pesticide on listed species and their designated critical habitat. During formal consultation, the Service(s) use the information in EPA’s final BE to develop their biological opinion (BiOp). Based on the outcomes of any relevant BiOps, EPA may determine that changes are necessary to one or more registrations to implement additional protections detailed in the BiOp(s).
EPA released an ESA workplan in April 2022, in which the Agency describes challenges and improvements to better protect federally listed species from possible effects of pesticides. In November 2022, EPA released an ESA Workplan Update (pdf) that details how EPA will pursue protections for non-target species, including listed species, earlier in the process for pesticide registration review and other FIFRA actions.
Learn more:
- EPA’s Workplan and Progress Toward Better Protections for Federally Listed Species
- Assessing Pesticides under the Endangered Species Act
Taking Action: Proposed Interim Decision, Interim Decision, Proposed Final Decision, and Final Decision
A registration review decision is EPA's determination of whether a pesticide meets or does not meet the statutory standard for registration; that is, whether taking into account the labeling, composition and packaging of the product, the pesticide can perform its intended function without unreasonable adverse effects on human health or the environment.
FIFRA defines the term ''unreasonable adverse effects on the environment'' to mean: ''(1) any unreasonable risk to man or the environment, taking into account the economic, social, and environmental costs and benefits of the use of any pesticide, or (2) a human dietary risk from residues that result from a use of a pesticide in or on any food inconsistent with the standard under section 408 of the Federal Food, Drug, and Cosmetic Act.''
In order for a pesticide to be registered, FIFRA requires that it not cause “unreasonable adverse effects on the environment.” This standard is defined in FIFRA as consisting of two parts. A pesticide may not cause an unreasonable risk to people or the environment, taking into account the economic, social, and environmental costs and benefits of the use of any pesticide nor, in the case of a pesticide resulting in residues in food, may it cause a “human dietary risk” that is not safe. EPA strives to meet the first prong of this statutory mandate through the implementation of a risk-benefit decision framework. For pesticides that are used on food or livestock feed, the safety analysis required for the second prong of the FIFRA standard imposes a risk-only framework. Similarly, benefits are not considered in decisions made under the Endangered Species Act.
Under the first prong, EPA considers the “economic, social, and environmental costs and benefits of the use of any pesticide” through an evaluation of risks and benefits (as appropriate). The risk findings in EPA risk assessments provide information on the “economic, social, and environmental” costs of the use of a pesticide by evaluating the potential adverse effects on human health and the environment from legal use of a pesticide. As appropriate, EPA also evaluates the benefits for the use of a pesticide. EPA benefit assessments provide information on the “economic, social, and environmental” benefits of the use of a pesticide, such as improvements in agricultural production, urban and recreational land management, and public health. When making registration and registration review decisions, EPA takes into account these findings and determines if the overall risk for use of the pesticide is “unreasonable.” If risks are identified as unreasonable, then EPA determines if additional mitigation measures can be implemented to reduce those risks. EPA considers the impacts of potential mitigation on the benefits of the pesticide, as appropriate. If identified risks cannot be reduced to no longer be unreasonable (after taking into account the benefits of the pesticide), EPA may determine that the pesticide does not meet the standards for registration.
Under the second prong, EPA may also determine that a pesticide does not meet the standard for registration if the use of the pesticide will result in a human dietary risk from residues pesticide in or on any food/feed that is inconsistent with the standard under section 408 of the Federal Food, Drug, and Cosmetic Act (FFDCA).
Proposed Decision (Interim or Final)
EPA will publish a Federal Register notice announcing the availability of a proposed registration review decision and will provide the public with a comment period of at least 60 days. The proposed decision and basis for the decision will be available in the pesticide's registration review docket.
In its proposed decision, among other things, EPA generally will:
- Present the findings of the Agency’s assessments, including risk assessments and any additional supporting documents (including benefit assessments). EPA will also present the results of formal ESA consultation, if needed and available;
- Propose any human health or ecological risk mitigation measures (changes to label requirements) necessary for the pesticide to be used in a manner that does not generally pose unreasonable risks, based on the published risk assessments and, if applicable, benefit assessments;
- Propose any mitigation measures (changes to label requirements) necessary to facilitate ESA consultation considering its 2022 ESA workplan, Balancing Wildlife Protection and Responsible Pesticide Use (pdf) and the ESA Workplan Update (pdf) ;
- State whether EPA believes additional data are needed and, if so, describe the data (a DCI may be issued to the registrant);
- Identify deadlines for completing any required actions.
EPA may issue a proposed interim decision when the Agency needs to conduct additional assessments such as a biological evaluation (for endangered species) or endocrine screening.
Interim Decision
EPA may issue, when appropriate, an interim registration review decision before completing a registration review. After considering any comments concerning the proposed interim decision, EPA may issue an interim registration review decision, including an explanation of any changes to the proposed interim decision and a response to significant comments. The Agency will publish a Federal Register notice announcing the availability of this decision.
The interim decision may, among other things,
- determine whether new or interim risk mitigation measures are necessary;
- identify data or information needed to complete the review (a DCI may be issued); and
- require the submission of updated labels.
If a registrant fails to take action required in an interim registration review decision, EPA may take appropriate regulatory action.
Final Decision
To conclude registration review, EPA will issue a final decision once all portions of the assessment have been completed, including, as appropriate, a listed-species assessment and any necessary ESA consultation with U.S. Fish and Wildlife Service and the National Marine Fisheries Service (the Services). EPA’s registration review final decisions will take into account the Endocrine Disruptor Screening Program screening consistent with the Federal Food, Drug, and Cosmetic Act § 408(p).
If a registrant fails to take action required in a registration review decision, EPA may take appropriate regulatory action.
